Description of
Parylene:
Parylene is the
trade name for a variety of polyxylylene
polymers marketed by several providers.
Parylene N is a polymer manufactured from
di-p-xylylene, a dimer synthesized from
p-xylylene. Di-p-xylylene, more properly
known as paracyclophane, is made from
p-xylylene in several steps involving
bromination, amination and elimination.
Parylene is a common
generic name for a unique series of polymers
based on paraxylene. The three most common
types of parylene are referred to as:
Parylene N, Parylene C, and Parylene D. In
actual usage, Parylene C is the greatly
predominant type of parylene used for almost
all types of applications and as such is
usually the type of material associated as
"Parylene." The basis for the parylene
family is the polyp-xylene monomer which
comprises Parylene N. Parylene C and D are
created by the substitution of a single
chlorine molecule (C) or two (double)
chlorine molecules (D). See Figure 1.
There are a number of
derivatives and isomers of Parylene, but
only a few are used commercially, e.g.,
Parylene N, Parylene C and Parylene D. This
article discusses the molecule which isn't
substituted, which produces Parylene N.
Heating paracyclophane in a partial vacuum
gives rise to a diradical species which
polymerizes when deposited on a surface.
Until the "monomer" comes into contact with
a surface it is in a gaseous phase and can
access the entire exposed surface. It has a
variety of uses. In electronics, chemical
vapor deposition at low pressure onto
circuit boards produces a thin, even
conformal polymer coating. Parylene coating
has very high electrical resistivity and
resists moisture penetration. It is used as
a dielectric in certain high-performance
capacitors for precision measurement. It has
uses in preserving archival paper.
Characteristics and
advantages
-
Hydrophobic,
chemically resistant
coating with good
barrier for inorganic
and organic media,
strong acids, caustic
solutions, gases and
water vapor.
- Outstanding
electrical isolation
with high tension strain
and low dielectric
constant
- A biostable,
biocompatible coating,
FDA permission
- micropore and pin
get-free starting from
0.2 µm layer thickness,
- Thin and transparent
coating with high gap
freedom of movement,
suitably for complex
arranged substrates also
on edges.
- Coating without
temperature load of the
substrates, coating
takes place at ambient
temperature in the
vacuum.
- Highly corrosion
resistant.
- Completely
homogeneous surface.
- Thermally stable up
to 220 °C, mechanically
stable from -200 °C to
+150 °C.
- Low mechanical
stresses.
- Resistant to
friction.
- Very low
permeability to gases.
- High electrical
impedance.
Typical applications
-
Dielectric coating
(e.g. Cores/coils).
- Hydrophobic coating
(e.g. biomedical hoses).
- Barrier layers (e.g.
for filter, diaphragms,
valves).
- Microwave
electronics.
- Sensors in rough
environment.
- Electronics for
space travel and
military.
- Corrosion protection
for metallic surfaces.
- Reinforcement of
micro-structures.
- Abrasion protection.
- Protection of
plastic, rubber, etc.
from harmful
environmental
conditions.
- Reduction of
friction (e.g. For
guiding catheters, also
acupuncture needles).
- Dissolving
deuterated polyethylene
for making nuclear
targets.
Additional
Advantages / Benefits
- MIL-I-46058C, Type
XY approved
- FDA approved -- USP
XXII, Class VI
bio-compatibility rating
- UL listed
- Completely pin-hole
free barrier coating
- Fully conformal on
any type of surface
material or design
- Inert transparent
polymer
- Meets NBC
requirements (AR70 /
AFR80-38 / Navinst
3400.2)
- Barrier to oxygen,
moisture, chemicals,
solvents, and carbon
dioxide
- Thermal mechanically
stable between -200ºC
and 150ºC
- Extremely high
dielectric 5,000 volts
per 0.001" minimum
- Excellent adhesion
properties
- Low stress coating
that does not form sites
prone to crack
initiation
- Low / minimal impact
on package cooling
- Hydrophobic
- Barrier to ionic and
moisture species
- Chemical and fungal
resistance
- Non-contaminating
coating and coating
process -- no solvents,
catalysts or other
by-products are
introduced during
coating
- Entire process is
accomplished at room
temperature, alleviating
temperature stress on
parts
- Particle
encapsulation /
immobilization
- No outgassing (NASA
approved)
- High reliability -
Suitable for military or
commercial applications
- Light weight -
compared to other
coatings
- Stress-free coatings
- Sensitive circuitry
unchanged by coating
- Low coefficient of
friction - Use as a dry
film lubricant
- Transparency - Thin
films can be of optical
quality
- Outgassing -
Virtually none
- Outstanding barrier
- Very low permeability
to moisture and gases
- Fungus and bacteria
resistance - Excellent
- Mechanical - High
tensile and yield
strength
- Radiation resistance
- Suitable for space
applications
The parylenes are formed
by the pyrolysis of a
di-p-xylene (dimer) in a
vacuum environment which is
then deposited on a cooler
(i.e. room temperature)
substrate under continuous
vacuum. Vapor phase
deposition of the parylene
polymer allows it to be
formed as a structurally
continuous film which is
truly conformal to the
design and structure of the
substrate upon which it is
being deposited. Parylene
can be effectively deposited
with excellent accuracy in
the thickness range of 0.1
mils to over 2 mils.
Figure 1
|
|
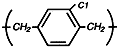
Parylene
C
The most widely
used dimer,
providing a
useful
combination of
properties, plus
a very low
permeability to
moisture,
chemicals, and
other corrosive
gases.
|
|
|
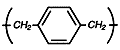
Parylene
N
Provides high
dielectric
strength and a
dielectric
constant that
does not vary
with changes in
frequency. Best
selection where
greater coating
protection is
required.
|
|
|
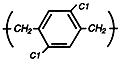
Parylene
D
Maintains its
physical
strength and
electrical
properties at
higher
temperatures.
|
|